Do you know what’s in your drinking water?
It may be more than you realize.
We disinfect our drinking water to protect us from organisms we can’t see. From boiling water to sand filters, water treatment dates back to the 18th century, and treating drinking water has saved countless lives. Right here in the Hudson Valley, Poughkeepsie, nicknamed “Sickly City” due illness from drinking water, was the first city in the U.S. to filter its drinking water in 1872.
First used in 1850, chlorine has provided the biggest improvement to drinking water quality. Chlorine is used to treat drinking water because of its ability to kill viruses and algae. It also has the ability to dissolve harmful chemicals that may be present in surface water, such as arsenic. Today, chlorine is the most widely used chemical to disinfect water.
However, concerns raised about health effects from chlorine byproducts formed during disinfection have led some experts to question the use of chlorine to produce safe drinking water.
What are disinfection byproducts, how are they formed, and how are they regulated?
When surface waters are treated for the purpose of drinking water, they are required by EPA regulations to be treated with chlorine and have residual value of chlorine throughout the system–basically, the chlorine level must be at least 0.2 mg/L when it reaches your faucet.
However, organic compounds that occur naturally in surface water react with the chlorine, creating disinfection byproducts (DBPs) that are harmful to humans. Bladder cancer is the biggest risk associated with exposure to DBPs. Research has yet to determine which DBP compounds are the most likely to cause bladder cancer.
Natural sources of organic compounds in surface waters include algae, soil, agricultural runoff, human sewage, and more. Each of these sources results in different organic compounds, causing the variation in DBPs.
DBPs were first discovered in the 1970s and now include over 700 compounds, many of which have yet to be studied.
Two groups of disinfection byproducts have been regulated by the EPA since 1998: trihalomethanes (THMs) and haloacetic acids. The Stage 1 and Stage 2 Disinfectants and Disinfection Byproducts Rules regulate a total of eleven DBPs.
The idea behind regulating THMs and HAAs was that doing so would promote the reduction of all DPBs, but scientists believe that the hundreds of unregulated DBPs may be more harmful to humans than the currently regulated compounds.
What’s being done to solve the DPB problem?
Prior to the discovery of the issues surrounding DBPs, when water first entered a water treatment facility, the first step in the treatment process was to apply chlorine. Water at the point of entry contained the highest level of organic compounds, meaning that a high number of DBPs were formed.
To combat this, water treatment facilities have attempted to lower the number of organic compounds in the water prior to treating with chlorine. The most common method is letting the water go through a sedimentation and filtration process where suspended particles containing organic compounds settle out of the water prior to disinfection.
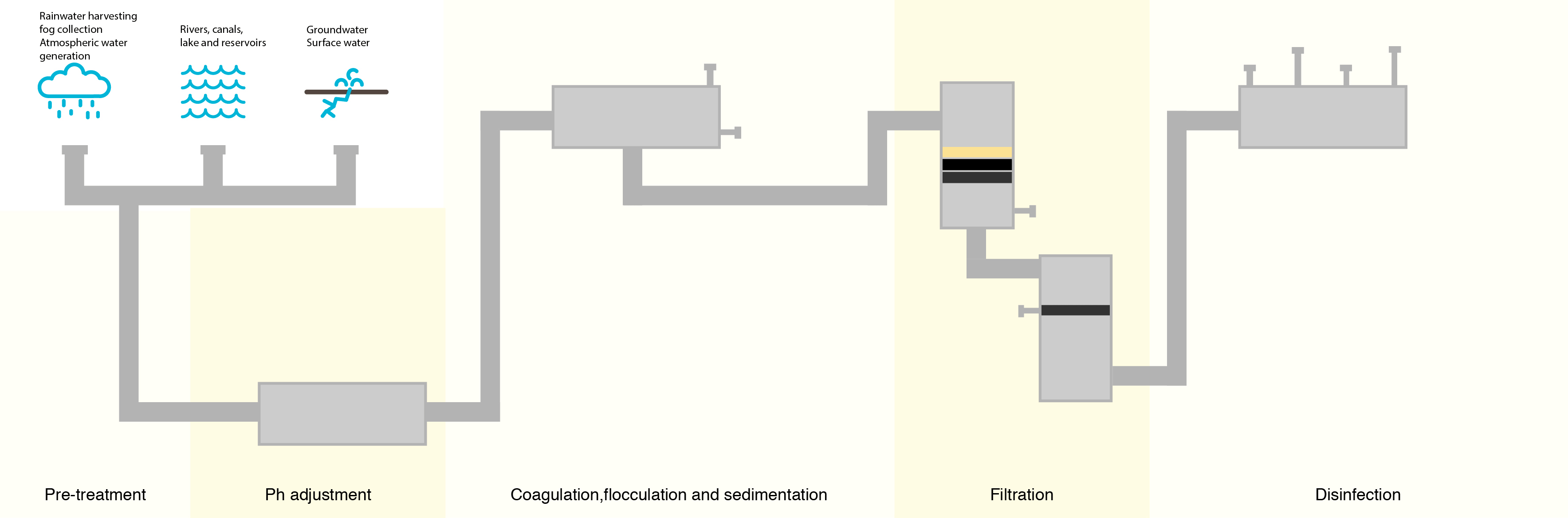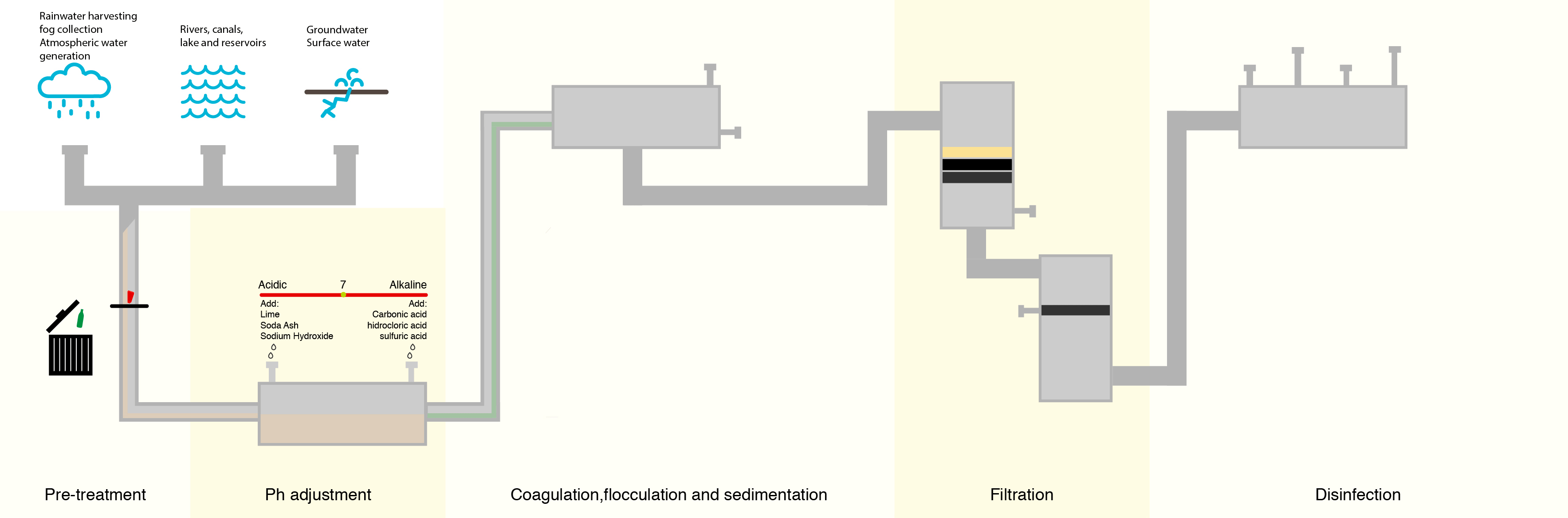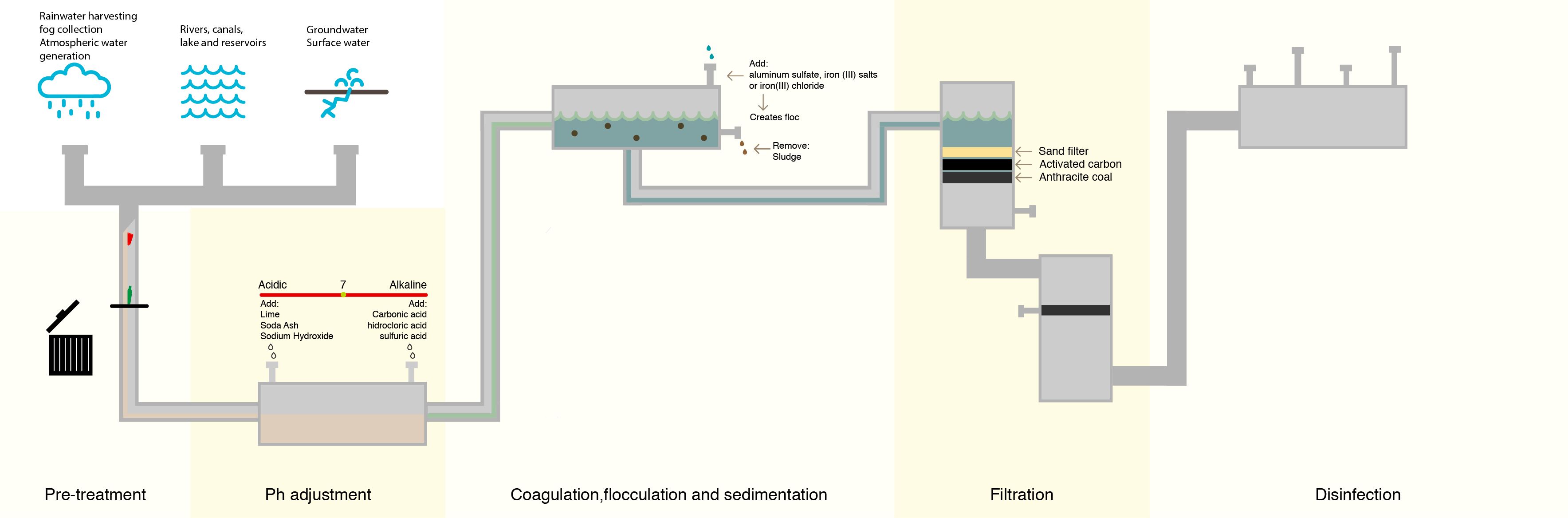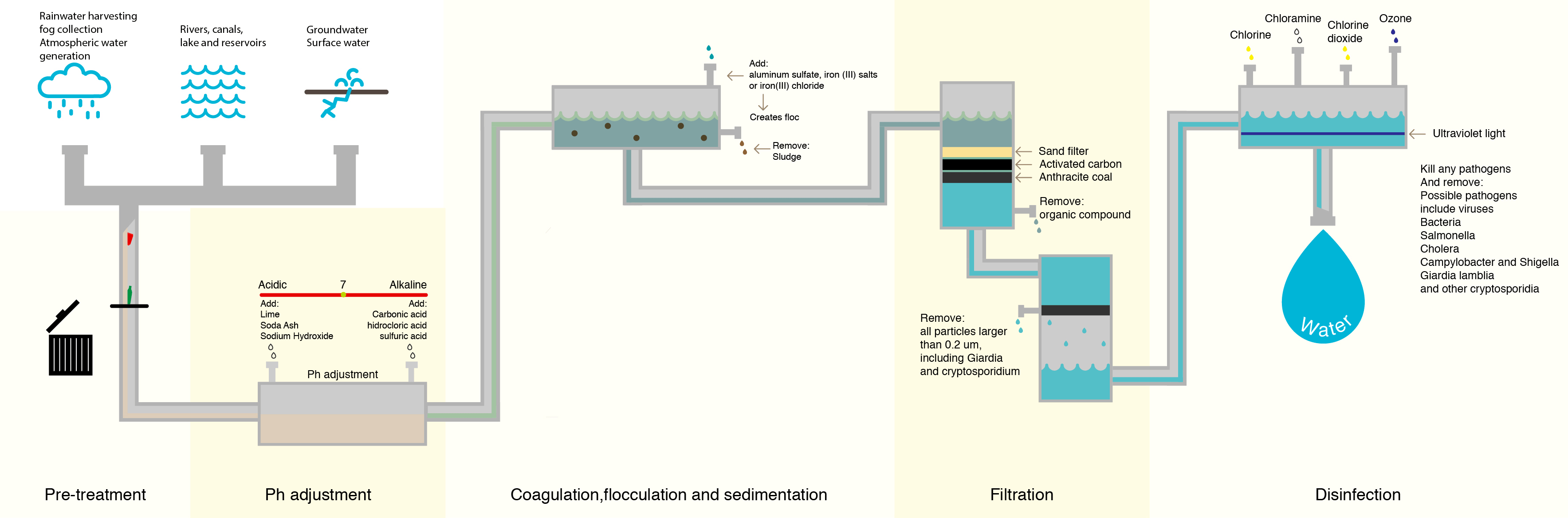
Although this process removes many of the denser organic compounds, it is not 100% effective, and organic compounds still remain.
Other water treatment options
Further treatment methods have been tested to reduce the level of regulated DBPs in drinking water. One seemingly simple alternative is the use of chloramine instead of chlorine.
Chloramine is formed when chlorine reacts with ammonia, and it’s regularly used as disinfectant in drinking water treatment. The biggest benefit to using chloramine is that fewer regulated DBPs are created during the water treatment process.
Another consideration is that DBP regulations have increased the cost of treating drinking water, and swapping out chlorine for chloramine is significantly cheaper than alternative options. Using chloramine means water treatment plants won’t violate DBP regulations and can spend the same amount on treatment costs as they were when previously using chlorine.
However, researchers have concluded that not only does the use of chloramine only reduce the level of DBPs that are currently regulated, it actually creates a subset of DBPs that are potentially more harmful than those that are currently regulated. Chloramine has also been proven to corrode metal pipes, causing increased levels of metal in drinking water.
Some more viable, but costly options include:
These methods would involve municipalities investing in new water treatment infrastructure, a cost that not all municipalities are able to afford.
What are the next steps?
Surface waters face risks from many sources of pollution, and DBPs are just one piece of the water treatment puzzle.
Additional scientific research is needed to fully understand the impacts of DBPs and identify the compounds that are most harmful. Regulations need to be updated and water treatment methods need to be tested to identify the method that’s most effective at removing harmful compounds.
This additional research is even more crucial in the face of climate change, which may alter the naturally occurring organic compounds in surface waters.
Find out the source of your drinking water. If your drinking water is provided by your municipality, then you can get access to your water quality reports. Your water quality report will tell you the level of regulated disinfection byproducts in your water. Be informed about your consumption.
Water is a precious resource that we cannot take for granted.


